NEW DELHI: Be careful while popping a pill for weight loss, muscle building or sexual enhancement. Several dietary supplements, most of which are widely available in India, have been found adulterated with unapproved and even banned pharmaceutical ingredients in the US with potential to cause serious health risks, a latest study published in 'JAMA Open' revealed.
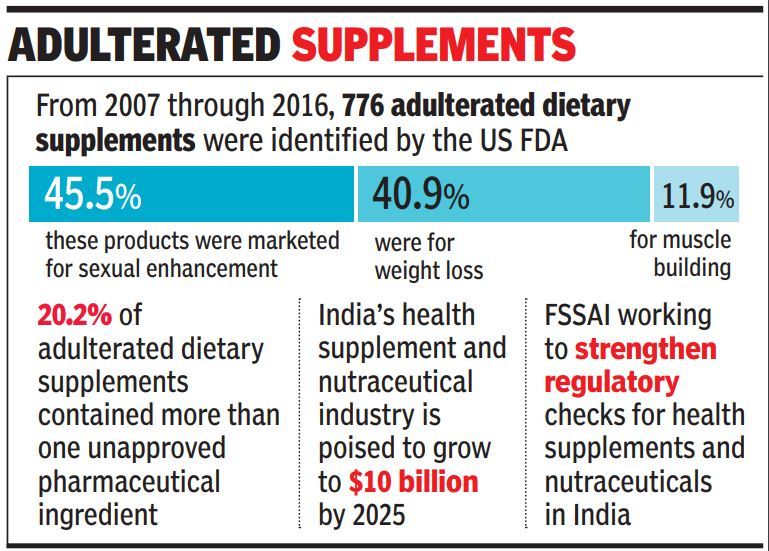
At least 776 dietary supplements sold over the counter in the US over a period of 10 years from 2007 to 2016 were found containing unapproved pharmaceutical ingredients such as sildenafil, sibutramine and synthetic steroids — which have potential to cause side-effects ranging from stroke to kidney failure and even death, say researchers, who extracted and analysed data from the US Food and Drug Administration's (US FDA) Center for Drug Evaluation and Research.
These products were commonly marketed for sexual enhancement, weight loss, or muscle building.
Doctors in India say the problem may be bigger here as most of the dietary supplements are easily available and widely used in India without any regulation and guidelines. Even the US regulator said it has been able to test only a portion of products available on the market.
"The issue is much more relevant in India because while this segment is largely regulated in the US, in our country it is completely unregulated. Dietary supplements in India are not tested or sampled by any authority and are easily available. The consumption is growing rapidly," says Dr Anoop Misra, chairman, Fortis C-Doc.
The dietary supplement segment which include nutraceuticals, foods for special dietary use and foods for special medical purpose poised to become a $10 billion industry by 2025. While rising income levels coupled with changing lifestyle have kindled demand for such products, lack of regulation and usage guidelines have made these products easily available over the counter and through online sales.
Of late, the growing demand and increasing availability of such products have also come to the notice of Indian regulatory authorities with the Food Safety and Standards Authority of India (FSSAI) working to strengthen the packaging norms for such products.
However, in the absence of wherewithal to do product sampling and testing, quality, efficacy and safety of such dietary supplements continue to be under question, says Dr Misra.
Another major grey area in this issue is the overlapping between food and drugs. While drugs or medicines are regulated by the Drugs Controller General of India (DCGI), food supplements come under the purview of FSSAI. Often to circumvent drug price regulation or stringent pharmaceutical norms, companies tweak their pharma formulations and launch their products as food supplements, bypassing regulatory approvals from the DCGI. The US FDA data shows, more than 20% of adulterated dietary supplements contained more than one unapproved pharmaceutical ingredients.

This comment has been removed by the author.
ReplyDeleteWhat really stuck with me is how casually these supplements are sold, despite the risks being so serious. The grey area between food and drugs feels dangerous, especially when people trust labels blindly. It almost makes processes like a drug and alcohol evaluation seem more transparent than supplement regulation.
ReplyDelete